
The aim of the “Sentinel” national surveillance program is to monitor the circulating respiratory viruses, including SARS-CoV-2 variants, and hence underpin public health actions.
In week 12/2021, the overall frequency of the SARS-CoV-2 B.1.1.7 variant in all samples sequenced increased to 73,5% (CI 69,8% – 77,2%, p<0,05). For the SARS-CoV-2 B.1.351 variant, we found an overall frequency of 18,4% (CI 15,2% – 21,6%, p<0,05) within the sequenced samples.
The representative sample was estimated, based on the number of positive cases in Luxembourg for week 12 (1690). The minimum sample size required to detect prevalence of B.1.1.7 (68%) reported in week 11, with an error margin of 5%, was estimated to be 280 specimens. This number corresponds to a coverage of 16,5 %, which exceeds the minimum coverage recommended by ECDC (10%). The sequencing results of week 12 are representative of the circulating variants in Luxembourg with a margin of error of 5%.
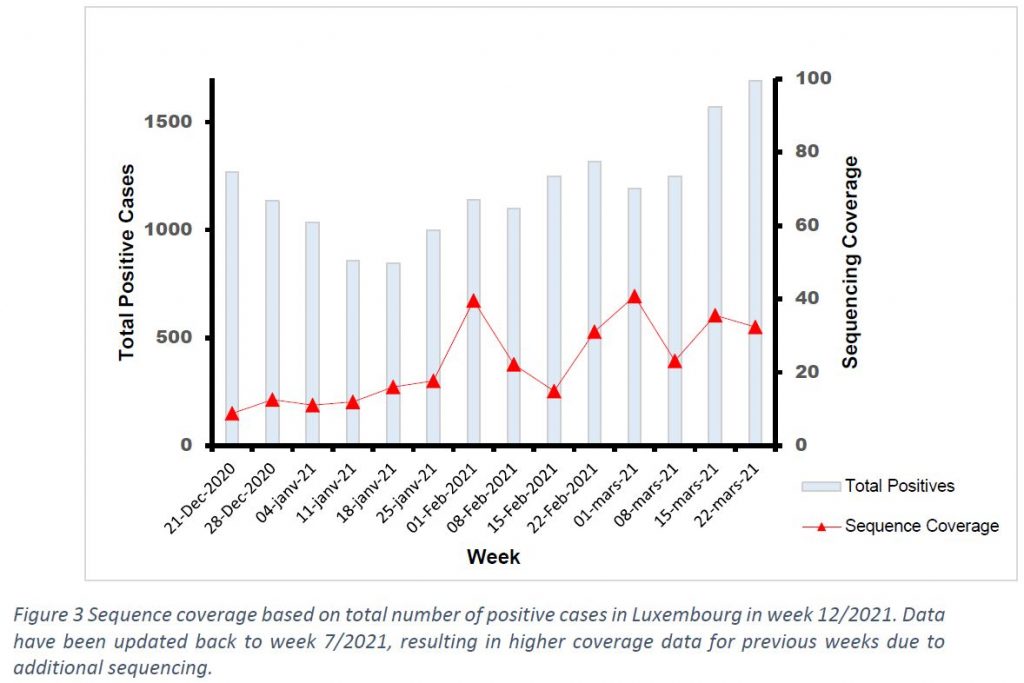
The total number of sequences performed this week was 689, with 548 specimens having been collected in the time frame of week 12/2021. The sequencing coverage this week was 32,4% from all positive cases in Luxembourg.
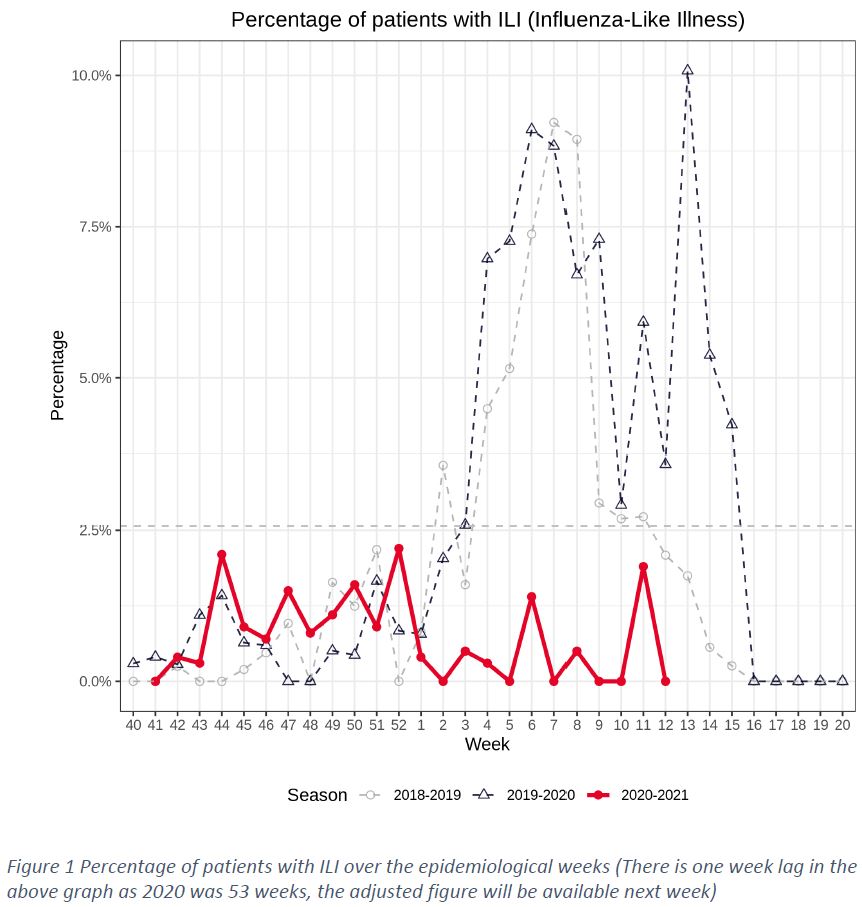
The “Sentinel” surveillance network reported 178 consultations in week 12 (22/MAR/2021 – 28/MAR/2021). There was no case of ILI1, as shown in Figure 1. The percentage of consultations for ARI2 was 19.6%.
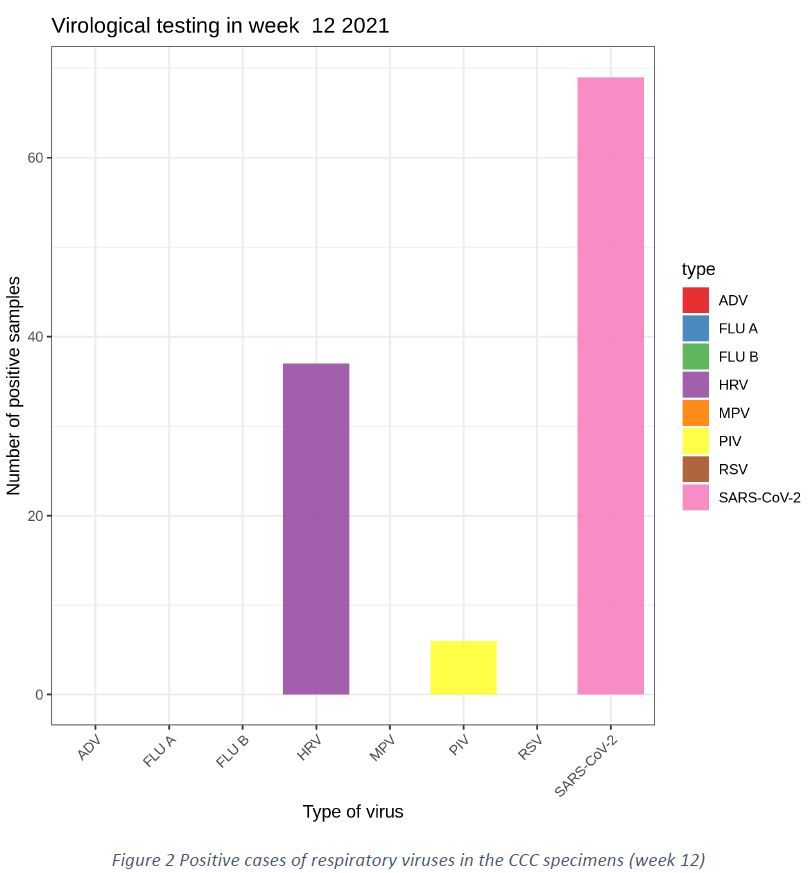
Patients presenting with ILI and ARI at the Covid Consultation Centre (CCC) in Luxembourg were tested using a respiratory virus panel (ADV = Adenovirus, FLU A = Influenza A, FLU B = Influenza B, HRV = Human Rhinovirus, MPV = Human metapneumovirus, PIV = Parainfluenza virus, RSV = Respiratory Syncytial Virus, SARS-CoV-2 = Severe acute respiratory syndrome coronavirus 2).
The SAR-COV-2 was the most prevalent respiratory virus detected in the “Sentinel” network, with 69 positive cases (28%). The wave of Human Rhinovirus (HRV) continued in week 12/2021, with 37 positive cases in 243 tests (15,2%). Sporadic cases of other respiratory viruses continued to appear, such as 6 cases of PIV in 243 tests (2,4%). No cases of Influenza A/B were detected, indicating absence of circulation of Influenza viruses in Luxembourg, as shown in Figure 2.
In Luxembourg, we have tested 243 samples from the Sentinel surveillance network, as compared to 1189 specimens tested in Europe, in the week 12/2021. None of these 1189 specimens tested positive for type A Influenza virus. The influenza epidemic in the European Region has usually reached its peak by this point of the year but, despite widespread and regular testing for influenza, reported influenza activity still remains at a very low level, likely due to the impact of the various public health and social measures, implemented to reduce transmission of SARS-CoV-2 (Source: FluNews Europe).
The National Reference Laboratory for Acute Respiratory Infections at LNS continues to improve the representativeness of the pool of sequenced specimens to reach real-time epidemiology, by implementing the following weekly sequencing activities:
The representative sequencing sample was based on the minimum number of specimens required to extrapolate prevalence of VOC variants with error rate of 5%. The representative sample was estimated based on the number of positive cases in Luxembourg for week 12 (1690). The minimum sample size required to detect prevalence of B.1.1.7 (68%) with an error margin of 5% was estimated to be 280 specimens. The calculation was based on a sample size calculation tool that uses the expected prevalence of the variant in the total population. (Population Proportion – Sample Size – Select Statistical Consultants (select-statistics.co.uk). This number represented a coverage of 16,5% which exceeds the minimum coverage recommended by ECDC (10%). The number of non-targeted specimens of Luxembourgish residents sequenced this week was 498. Therefore, our sequencing results this week are representative of the circulating variants in Luxembourg.
The starting material used for sequencing is respiratory specimens (nasopharyngeal or oropharyngeal swabs) that have already been tested positive by RT PCR.
The LNS sequencing data sharing strategy includes sharing of the sequencing data with GISAID EpiCov database (www.gisaid.org ) on a periodic basis.
Last week the microbial genomics platform at the LNS sequenced 548 specimens having been collected in week 12/2021. This represents 32,4% of the new infections reported in Luxembourg in week 12/2021. Among the 548 specimens, 29 specimens were reported to be part of a cluster or outbreak investigation, and 21 specimens were from non-residents. This leads to 498 specimens, collected in week 12, and being the representative population sequencing sample. In the population representative sample of residents, the frequency of B.1.1.7 and B.1.135 was 74,1% and 18,1% respectively.
The population sequencing coverage in week 12/2021 was 32,4% (Figure 3). Based on statistical inference, the frequency of the reported variants in week 12/2021 is representative of the circulating variants in Luxembourg with a margin of error of 5%.
Lineages (variants) have been assigned based on Rambaut et al by means of Phylogenetic Assignment of Named Global Outbeak LINeages (pangolin) software (v2.3.6, pangoLEARN version 2021-03-29).
The lineage nomenclature system that we use is the one proposed by Rambaut et al. that focuses on actively circulating virus lineages (https://cov-lineages.org ).
In week 12/2021, in the population representative sample, after removal of cluster samples, and excluding specimens collected from non-residents, there were 13 variants, with the main three variants being B.1.1.7 (74,1%, CI 70,2% – 77,9%), B.1.351 (18,1%, CI 14,7% – 21,5%), followed by B.1 (1,6%, CI 0,5% – 2,7%), as shown in Figure 4.
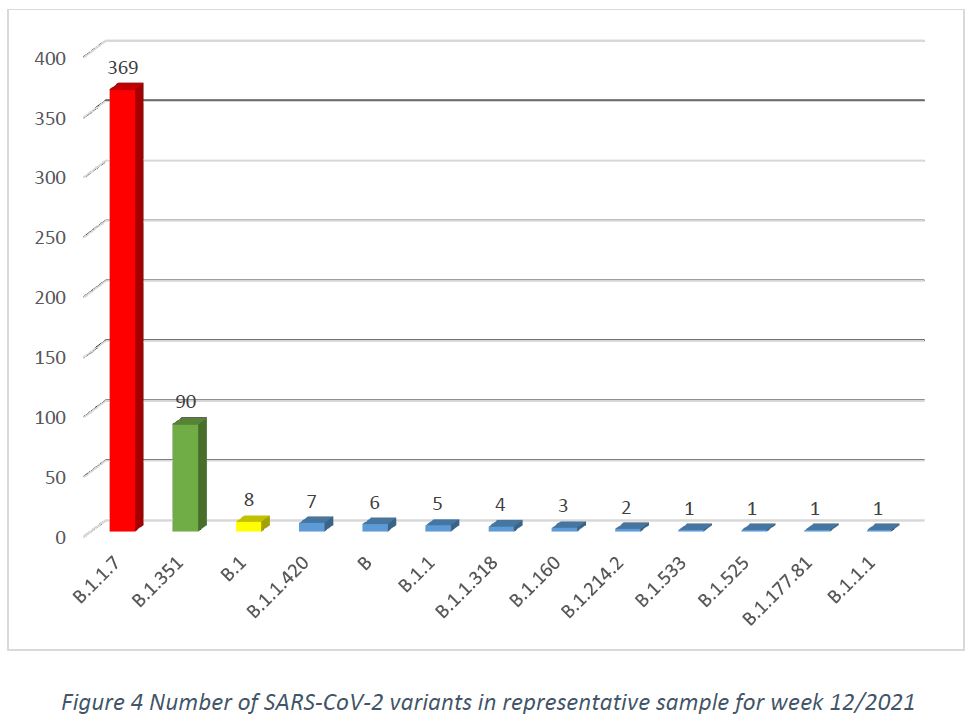
Among specimens collected within the week 12/2021, 403 cases of the B.1.1.7 variant have been detected, representing 73,5% of the specimens in the week’s sequencing pool (by comparison, the week 11/2021 pool had shown a frequency of 67,8% of this variant, including additional specimens having been sequenced from previous weeks). The total case count of sequenced variant B.1.1.7 was 2115 by week 12/2021. The earliest collection date for this variant remains 19/DEC/2020 and the latest is 28/MAR/2021.
In the collection period of week 12/2021, 101 cases of the South African variant B.1.351 have been detected, representing 18,4% of the specimens in the week’s sequencing pool (by comparison, the week 11/2021 pool had shown 22,9% of this variant, including additional specimens having been sequenced from previous weeks). The total case count of sequenced variant B.1.351 was 554 by week 12/2021. The earliest collection date for this variant remains 11/JAN/2021 and the latest is 28/MAR/2021.
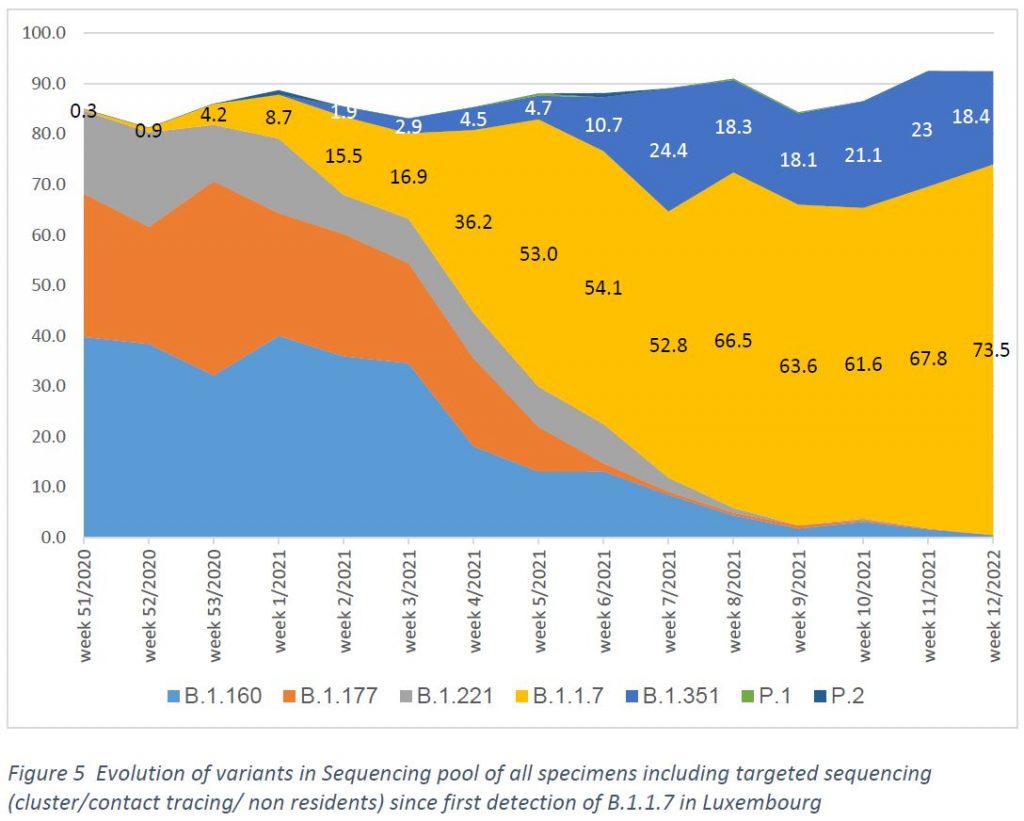
In week 12/2021 one additional case has been detected for B.1.525, but not any of the other variants of concern (P.1, A.23.1) (Figure 5).
Lineage B.1.1.7 is characterized by several spike protein mutations, including N501Y, H69/V70del and P861H. The variant seems to have a considerable epidemiological impact, as it has a higher transmissibility rate.
Lineage B.1.351 holds numerous spike protein mutations, of which three are located in the receptor binding domain (K417N, E484K and N501Y), and are therefore relevant for antibody binding. As for B.1.1.7, a higher transmissibility rate and viral loads seem to be associated with this variant. Due to the K417N and E484K mutations, an impact on vaccination efficacy and possibility of reinfection is subject to scientific investigation.
Lineage P.1 (descendent of B.1.1.28), initially found in the Amazon region, has a similar mutation profile as the South African variant, including E484K and N501Y. Concerns are, as for the South African variant, higher transmissibility and a decreased protection by neutralizing antibodies.
Lineage B.1.525 carries several mutations of biological significance, including E484K, Q677H and F888L. It does not carry N501Y, but a set of deletions similar to the B.1.1.7 variant.
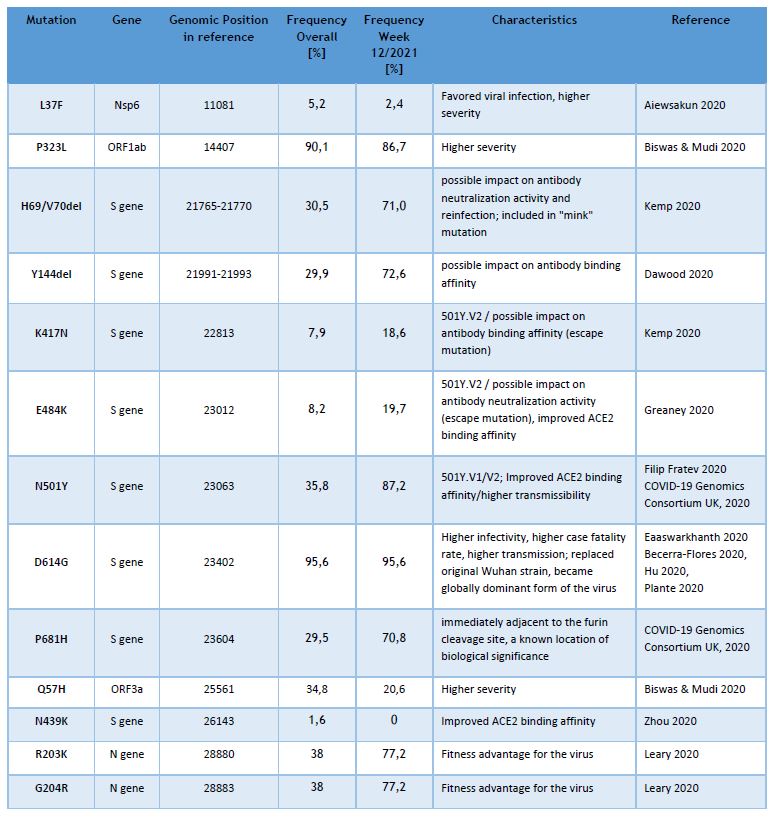
Currently the LNS genomic surveillance program – independently from lineage calling – notes the occurrence of 13 different known SARS-CoV-2 mutations, assumed to have clinical and epidemiological relevance. The list of observed mutations is being updated continually, based on the appearance and prevalence of SARS-CoV-2 variants.
The following table provides the overall frequencies of these mutations, detected in the lineage-assignable genome sequences, analyzed between 01/SEP/2020 and 22/MAR/2021 (N=7040), as well as the frequencies in week 12/2021.
Genomic sequencing of SARS-CoV-2. A guide to implementation for maximum impact on public health. WHO, 8 January 2021.
COVID-19 data portal. 2020 (https://www.covid19dataportal.org/sequences )
J Hadfield et al. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics 2018;34:4121-4123
A Rambaut et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol 2020;5:1403-1407
https://github.com/cov-lineages/pangolin
Y Guangchuang et al. ggtree: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods in Ecology and Evolution 2017;8:28-36
For more information on lineages visit: https://cov-lineages.org
For more information and statistics on Covid-19 infections in Luxembourg visit: https://covid19.public.lu/en.html