
The Sentinel Surveillance Network identified 2 cases of influenza-like illnesses, which remains below the recommended threshold for the interepidemic season, according to the European Center for Disease Prevention and Control (ECDC) guidelines.
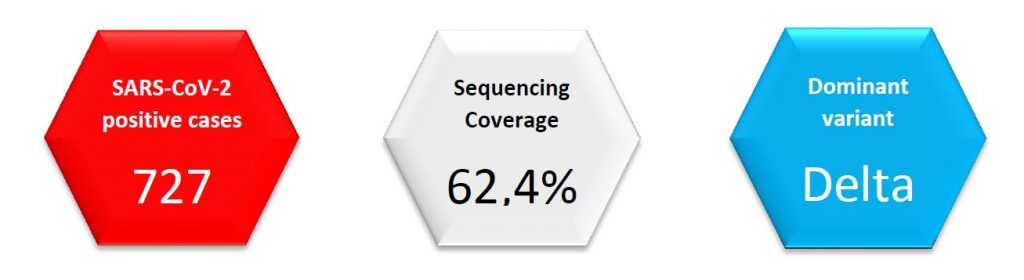
Regarding SARS-CoV-2 genomic surveillance, the Laboratoire national de santé analysed 454 specimens in week 28/2021 (from 727 total cases in the Great Duchy of Luxembourg, 62,4%). This exceeds the minimum coverage (10%) and sample size recommended by the ECDC (which is 45,3% in our current epidemiological situation).
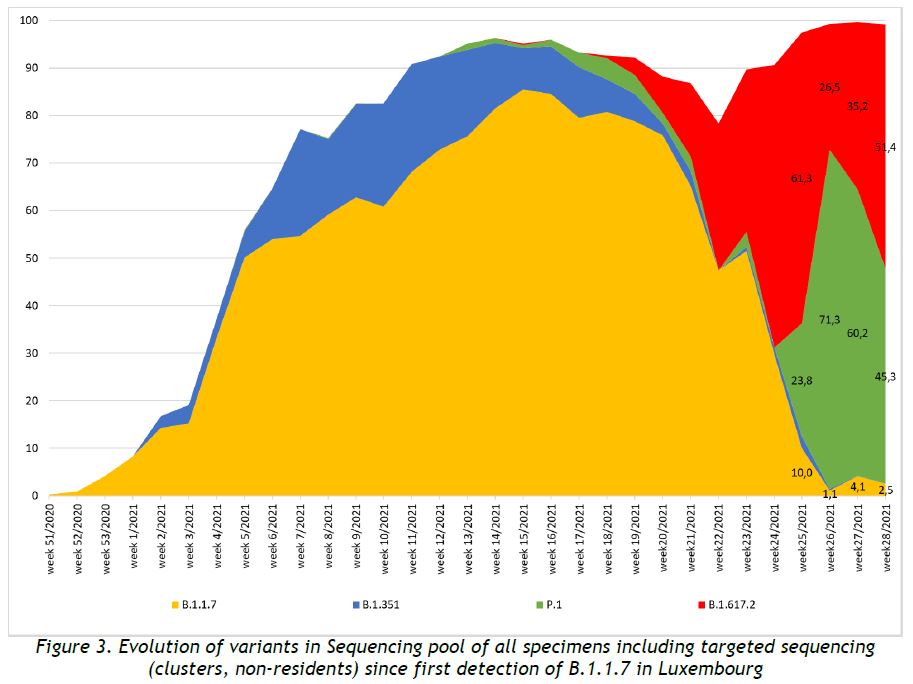
Community case surveillance revealed an increase of Delta variant cases, which have been on an increasing trend over the last five weeks. Currently, the Delta variant remains the dominant variant in Luxembourg.
Target group surveillance showed 49 VOCs within hospitalized cases (51,0% Delta and 46,9% Gamma) and all cluster cases were linked to VOCs (80,6% Delta, 19,4% Gamma). Additionally, 14 reinfection or post-vaccination breakthrough cases linked to VOCs were identified.
The Laboratoire national de santé, as National Reference Laboratory for Acute Respiratory Infections in Luxembourg, performs close surveillance on respiratory viruses, with a special focus on SARS-CoV-2. There are currently three projects:
The ReViLux provides updates on the first two projects.
The Sentinel Surveillance Network aims at monitoring the circulating respiratory viruses, including SARS-CoV-2, and hence underpin public health actions. Following the WHO[1] and ECDC[2] guidance, it focuses on cases of acute respiratory infection (ARI) and influenza-like illness (ILI).
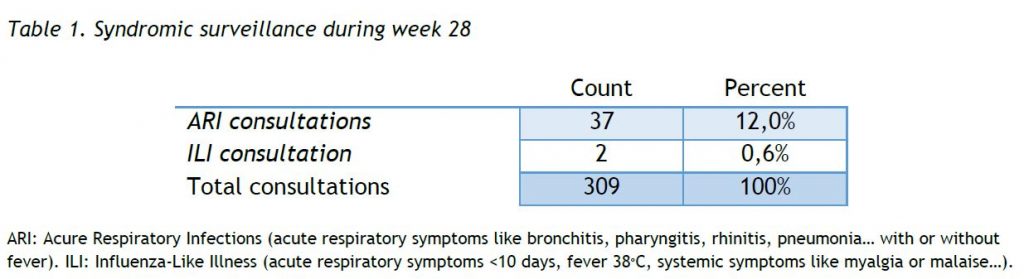
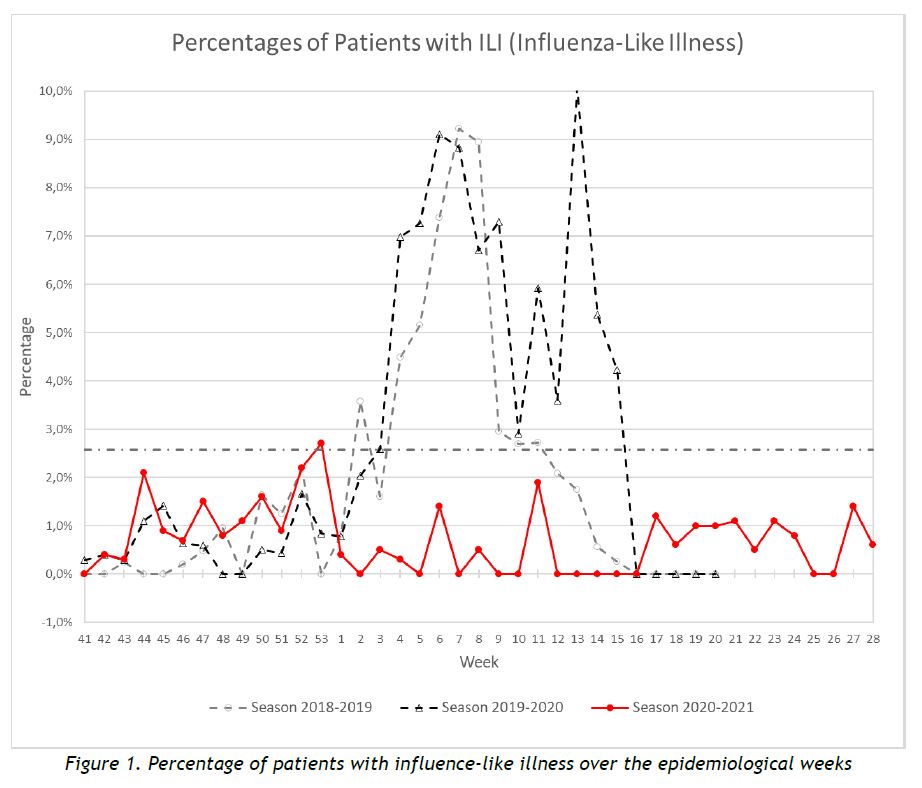
Results of syndromic surveillance during week 28 (12 July 2021 – 18 July 2021) are displayed in Table 1 and the history of ILI consultations since the 2018-2019 season is shown in Figure 1. The percentage of ILI remains below the threshold for the interepidemic season, according to the ECDC.
Regarding the virological surveillance, no data is available for week 28.
[1] World Health Organization
[2] European Centre for Disease Prevention and Control
The National Reference Laboratory for Acute Respiratory Infections at LNS receives SARS-CoV-2 -positive samples for (nasopharyngeal or oropharyngeal swabs analysed by RT-PCR) from the national network of laboratories and proceeds as follows:
The representative sample of community cases is a selection from all cases to detect emerging SARS-CoV-2 variants and early increases in their incidence and transmission within the community in Luxembourg. This sample is selected according to ECDC guidelines.
In week 28, 727 new cases were registered in Luxembourg; hence, the minimum sample size required to detect a 2.5% incidence is estimated to be 329 specimens (45,3%). The number of non-targeted specimens from Luxembourgish residents successfully sequenced this week was 425 (58,5%), which exceeds the minimum coverage (10%) and minimum sample size (329) recommended by ECDC.
The LNS shares its sequencing results with GISAID EpiCov database (www.gisaid.org) periodically. SARS-CoV-2 lineages (variants) have been assigned based on Rambaut et al. using Phylogenetic Assignment of Named Global Outbreak LINeages (pangolin) software (v3.1.5, pangoLEARN version 2021-06-15). The ReViLux continues to use the Pango nomenclature, in addition to the WHO nomenclature, to allow easier visualization of links between any evolving variants and their ancestor (https://cov-lineages.org). See nomenclature equivalences in Annex 1.
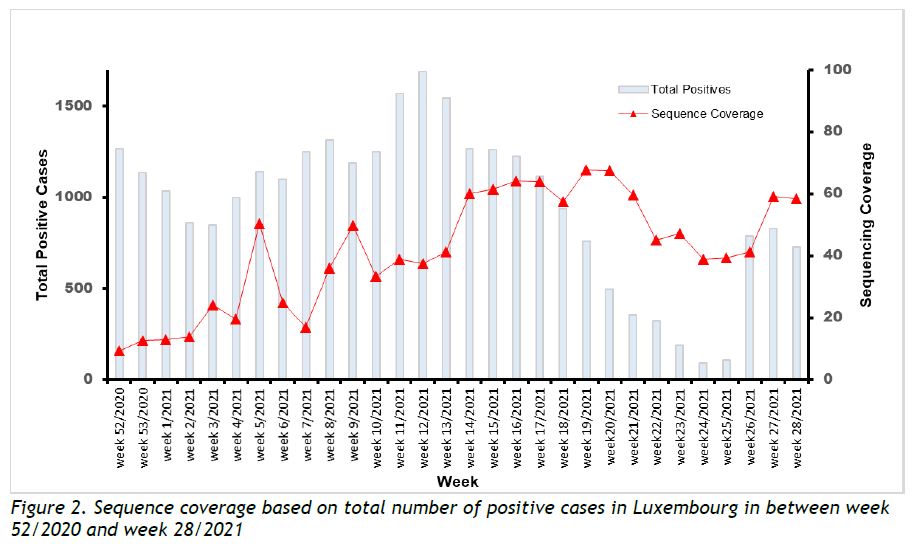
Last week, the microbial genomics platform at the LNS sequenced 559 specimens, with 475 having been collected in week 28/2021. Among the latter, 31 specimens were reported to be part of a cluster or vaccine failure investigation, and 21 specimens were from non-residents (2 specimens overlapping). The number of non-targeted specimens from Luxembourgish residents successfully sequenced this week was 425 (58,5% coverage of 727 total cases) (see coverage trend in Figure 2).
The evolution of variants over the weeks is shown in Figure 3. Delta variant showed a constant increase, in absolute values, during the last five weeks, and has become again the dominant variant.
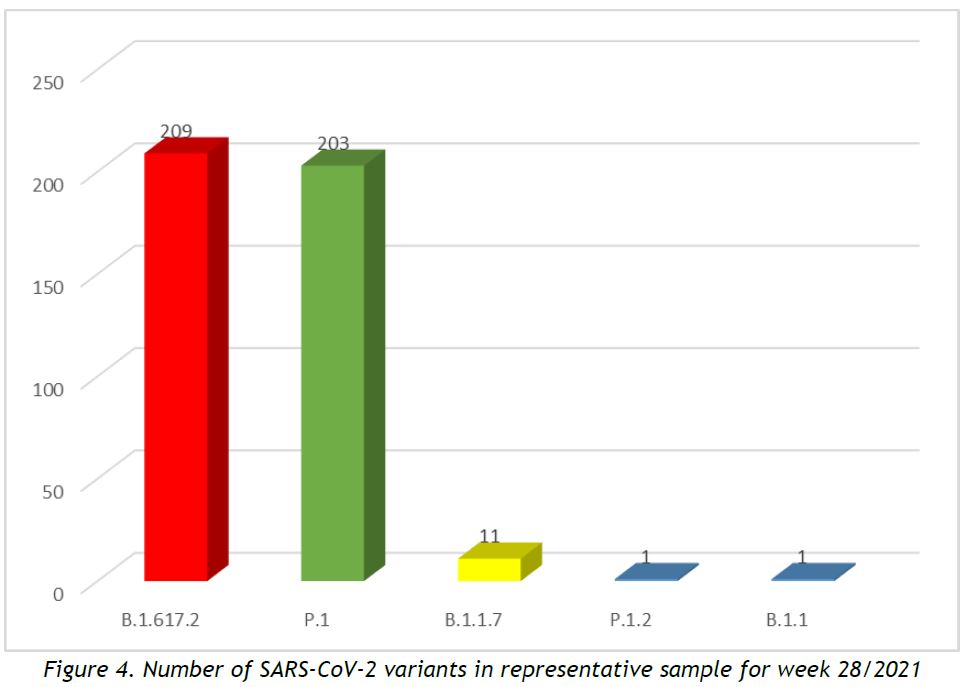
In week 28/2021, 5 circulating SARS-CoV-2 variants were detected within our representative sequencing pool, after removal of cluster specimens, and excluding specimens collected from non-residents, as shown in Figure 4. The most prevalent lineages are displayed in Table 2 and information about the lineages is provided in Annex 2.

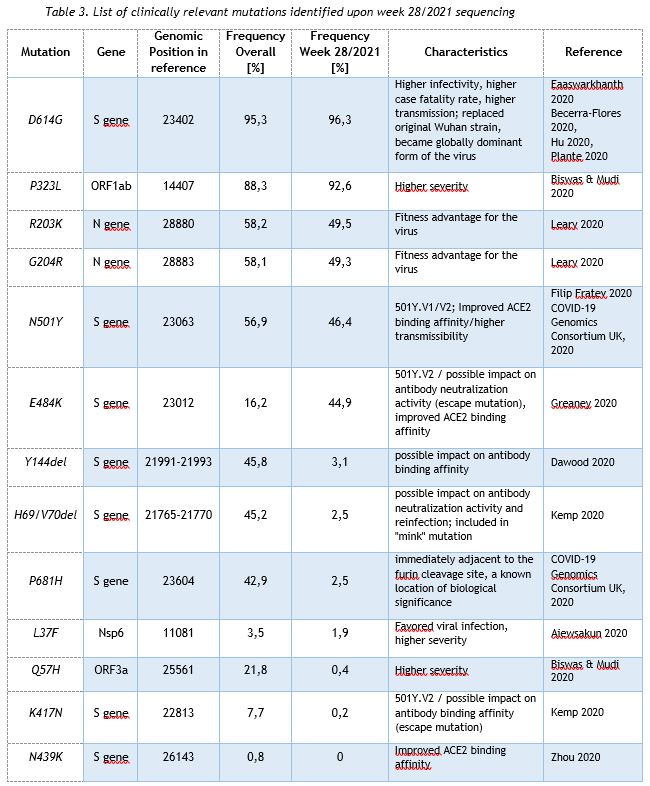
In addition to the surveillance of SARS-CoV-2 variants, the LNS monitors the occurrence of SARS-CoV-2 mutations assumed to have a clinical and epidemiological relevance. Currently, 13 mutations are being observed, and this list is updated continually.
Table 3 provides the overall frequencies of these mutations, detected in the lineage-assignable genome sequences, analyzed between 1 Sep 2020 and 11 Jul 2021 (N=14977), as well as the frequencies in week 28/2021.
COVID-19 Data Portal – accelerating scientific research through data. (2021). Retrieved 21 July 2021, from https://www.covid19dataportal.org/sequences
European Centre for Disease Prevention and Control. Guidance for representative and targeted genomic SARS-CoV-2 monitoring – 3 May 2021. ECDC : Stockholm ; 2021
Genomic sequencing of SARS-CoV-2: a guide to implementation for maximum impact on public health. Geneva: World Health Organization; 2021.
GitHub – cov-lineages/pangolin: Software package for assigning SARS-CoV-2 genome sequences to global lineages. (2021). Retrieved 21 July 2021, from https://github.com/cov-lineages/pangolin
Hadfield, J., Megill, C., Bell, S., Huddleston, J., Potter, B., & Callender, C. et al. (2018). Nextstrain: real-time tracking of pathogen evolution. Bioinformatics, 34(23), 4121-4123. doi: 10.1093/bioinformatics/bty407
Instituto Nacional de Saúde Doutor Ricardo Jorge. Diversidade genética do novo coronavírus SARS-CoV-2 (COVID-19) em Portugal. Retrieved 26 July 2021, from https://insaflu.insa.pt/covid19/
Rambaut, A., Holmes, E., O’Toole, Á., Hill, V., McCrone, J., & Ruis, C. et al. (2020). A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nature Microbiology, 5(11), 1403-1407. doi: 10.1038/s41564-020-0770-5
Robert Koch Institut. Aktueller Lage-/Situationsbericht des RKI zu COVID-19. Retrieved 26 July 2021, from https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Gesamt.html;jsessionid=69BC18053F9591C56EB148E463103DB7.internet101
Santé publique France. Enquêtes Flash : évaluation de la circulation des variants du SARS-CoV-2 en France. Retrieved 26 July 2021, from https://www.santepubliquefrance.fr/etudes-et-enquetes/enquetes-flash-evaluation-de-la-circulation-des-variants-du-sars-cov-2-en-france
Sciensano. COVID-19 – Bulletin épidémiologique hebdomadaire (23 juillet 2021). Retrieved 26 July 2021, from https://covid-19.sciensano.be/fr/covid-19-situation-epidemiologique
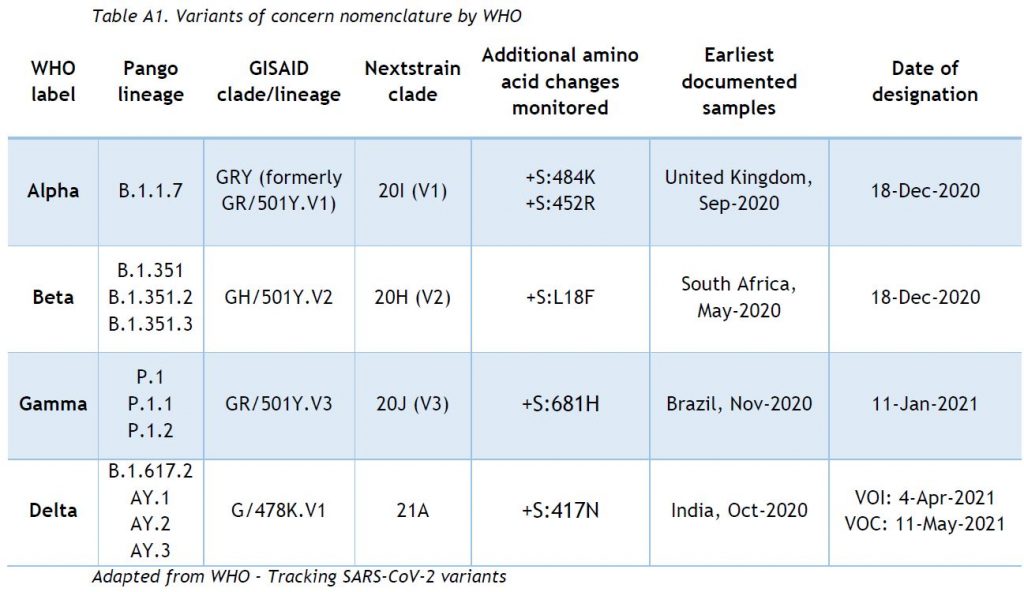
The ReViLux continues to use the (Pango) system to allow easier visualisation of links between any evolving variants and their ancestor. Equivalence for most frequently used VOC nomenclatures are shown in Table A1 (adapted from WHO).
Lineage B.1.1.7 is characterized by several spike protein mutations, including N501Y, H69/V70del and P861H. The variant seems to have a considerable epidemiological impact, as it has a higher transmissibility rate.
Lineage B.1.351 holds numerous spike protein mutations, of which three are located in the receptor binding domain (K417N, E484K and N501Y), and are therefore relevant for antibody binding. As for B.1.1.7, a higher transmissibility rate and viral loads seem to be associated with this variant. Due to the K417N and E484K mutations, an impact on vaccination efficacy and possibility of reinfection is subject to scientific investigation.
Lineage P.1 (descendent of B.1.1.28), initially found in the Amazon region, has a similar mutation profile as the South African variant, including E484K and N501Y. Concerns are, as for the South African variant, higher transmissibility and a decreased protection by neutralizing antibodies.
Lineage B.1.525 carries several mutations of biological significance, including E484K, Q677H and F888L. It does not carry N501Y, but a set of deletions similar to the B.1.1.7 variant.
Lineage B.1.617 is a variant first detected in India and was designated “Under Investigation” on 1st April 2021 by Public Health England. It contains a number of spike mutations associated with antigenic escape or found in other variants of concern, including L452R, E484Q and P681R. Subtype B.1.617.2 does not carry S:E484Q and seems to be more transmissible than B.1.1.7 (increasing confidence). Neutralization studies show reductions in cross-neutralizing activity between B.1.1.7 and B.1.351.